You are using a browser that is not supported by this site. The site will not function properly. Please switch to the latest version of a supported browser such as Chrome, Safari, Edge, or Firefox to use this site.
Offering options to help make living with a pump easier
United Therapeutics’ dedication to innovation means that you have choices when it comes to pump treatment. Once your doctor prescribes Remodulin, they’ll help you decide which route of administration and pump fits your needs. Together, you can review each pump option to see which one is best for you.
Remember, many people have successfully incorporated Remodulin pump therapy into their lives—and so can you!
How is Remodulin administered?
Remodulin can be administered either subcutaneously or intravenously through 5 different pump options
Subcutaneous (SC) pump options
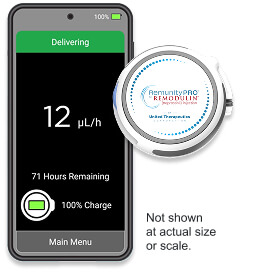
RemunityPRO™
A small, user-friendly pump for patients with PAH designed to fit more easily into your life*
Learn More
First generation of Remunity®
A small, discreet, water-resistant* pump designed to simplify the delivery of Remodulin
Learn More*The first generation of Remunity and RemunityPRO pumps can tolerate immersion in water to depths of up to 8 feet for 30 minutes and 12 feet for up to 3 minutes. The remote, battery charger, and AC adapter are NOT waterproof and should be kept clean and dry. The patient’s subcutaneous site should be protected. Patients should talk to their healthcare provider before swimming or engaging in water-based activities.

CADD-MS® 3 ambulatory infusion pump
A traditional all-in-one pump
LIMITED SUPPLY AVAILABLE
Learn MoreIntravenous (IV) Pump Options

CADD-LEGACY® ambulatory infusion pump
A widely used and portable IV pump
LIMITED SUPPLY AVAILABLE
Learn MoreWhen starting Remodulin, all patients will receive 2 pumps so they always have a backup pump available if needed.
Sign up to get support emails
Become a member of the Remodulin Support Program to get helpful tips, resources, and more delivered straight to your inbox.
Remodulin® (treprostinil) Injection
Important Safety Information for Remodulin
Before you take Remodulin, tell your healthcare provider if you:
- Have other medical conditions or take other medicines that may affect your use of Remodulin by increasing the risk of side effects or decreasing the drug’s effectiveness.
- Have liver problems. Your Remodulin dose may need to be adjusted if you have liver problems.
- Have low blood pressure or bleeding problems.
- Are taking gemfibrozil (for high cholesterol), rifampin (for infection) or other drugs that affect liver enzymes. Your doctor may need to adjust your Remodulin dosage.
- Are pregnant, breastfeeding, or planning to become pregnant. It is not known if Remodulin will harm your unborn baby or if Remodulin passes into your breast milk.
What are the serious side effects of Remodulin?
- Continuous intravenous (IV) infusions of Remodulin delivered using an external infusion pump, with a tube placed in a central vein within the chest, are associated with the risk of blood stream infections and sepsis, which may be fatal. Therefore, continuous subcutaneous (SC) infusion delivered just beneath the skin is the preferred type of delivery.
- Worsening of PAH symptoms. Do not stop taking or greatly reduce your Remodulin dose without consulting your doctor.
- Low blood pressure (symptomatic hypotension). If you have low blood pressure or are taking drugs that lower your blood pressure, the risk of low blood pressure is increased.
- Bleeding problems. Remodulin may increase the risk of bleeding in people who take blood thinners (anticoagulants).
What are the possible side effects of Remodulin?
- In clinical studies of SC infusion of Remodulin, most people experienced infusion site pain and infusion site reaction (redness, swelling, and rash). These symptoms were sometimes severe and sometimes required treatment with narcotics or discontinuation of Remodulin.
- IV infusion of Remodulin delivered through an external pump has been associated with the risk of blood stream infections, arm swelling, tingling sensations, bruising, and pain.
- The most common side effects seen with either SC or IV Remodulin were headache, diarrhea, nausea, rash, jaw pain, widening of the blood vessels (vasodilatation), and swelling from fluid retention (edema). These are not all the possible side effects of Remodulin. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at www.fda.gov/MedWatch or call 1-800-FDA-1088.
You may report side effects to the FDA at www.fda.gov/MedWatch or call 1-800-FDA-1088.
What is Remodulin?
Remodulin is a prescription medication used to treat adults with pulmonary arterial hypertension (PAH; WHO Group 1), which is high blood pressure in the arteries of your lungs. Remodulin can reduce symptoms associated with exercise. Remodulin was studied mainly in patients with NYHA Functional Class II-IV symptoms. It is not known if Remodulin is safe and effective in children.
In people with PAH who need to switch from epoprostenol, Remodulin is approved to slow the worsening of symptoms.
The risk information provided here is not comprehensive. To learn more about Remodulin, talk with your healthcare provider. Please see Full Prescribing Information at www.remodulin.com or call Customer Service at 1-844-UNITHER (1-844-864-8437).
To learn more about Remodulin, talk with your healthcare provider. Please see Full Prescribing Information at www.remodulin.com or call Customer Service at 1-844-UNITHER (1-844-864-8437).
REMISIconFEB2025
PAH=pulmonary arterial hypertension; WHO=World Health Organization.
Remodulin® (treprostinil) Injection
Important Safety Information for Remodulin
Before you take Remodulin, tell your healthcare provider if you:
- Have other medical conditions or take other medicines that may affect your use of Remodulin by increasing the risk of side effects or decreasing the drug’s effectiveness.
- Have liver problems. Your Remodulin dose may need to be adjusted if you have liver problems.
- Have low blood pressure or bleeding problems.
- Are taking gemfibrozil (for high cholesterol), rifampin (for infection) or other drugs that affect liver enzymes. Your doctor may need to adjust your Remodulin dosage.
- Are pregnant, breastfeeding, or planning to become pregnant. It is not known if Remodulin will harm your unborn baby or if Remodulin passes into your breast milk.
What are the serious side effects of Remodulin?
- Continuous intravenous (IV) infusions of Remodulin delivered using an external infusion pump, with a tube placed in a central vein within the chest, are associated with the risk of blood stream infections and sepsis, which may be fatal. Therefore, continuous subcutaneous (SC) infusion delivered just beneath the skin is the preferred type of delivery.
- Worsening of PAH symptoms. Do not stop taking or greatly reduce your Remodulin dose without consulting your doctor.
- Low blood pressure (symptomatic hypotension). If you have low blood pressure or are taking drugs that lower your blood pressure, the risk of low blood pressure is increased.
- Bleeding problems. Remodulin may increase the risk of bleeding in people who take blood thinners (anticoagulants).
What are the possible side effects of Remodulin?
- In clinical studies of SC infusion of Remodulin, most people experienced infusion site pain and infusion site reaction (redness, swelling, and rash). These symptoms were sometimes severe and sometimes required treatment with narcotics or discontinuation of Remodulin.
- IV infusion of Remodulin delivered through an external pump has been associated with the risk of blood stream infections, arm swelling, tingling sensations, bruising, and pain.
- The most common side effects seen with either SC or IV Remodulin were headache, diarrhea, nausea, rash, jaw pain, widening of the blood vessels (vasodilatation), and swelling from fluid retention (edema). These are not all the possible side effects of Remodulin. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at www.fda.gov/MedWatch or call 1-800-FDA-1088.
What is Remodulin?
Remodulin is a prescription medication used to treat adults with pulmonary arterial hypertension (PAH; WHO Group 1), which is high blood pressure in the arteries of your lungs. Remodulin can reduce symptoms associated with exercise. Remodulin was studied mainly in patients with NYHA Functional Class II-IV symptoms. It is not known if Remodulin is safe and effective in children.
In people with PAH who need to switch from epoprostenol, Remodulin is approved to slow the worsening of symptoms.
The risk information provided here is not comprehensive. To learn more about Remodulin, talk with your healthcare provider. Please see Full Prescribing Information at www.remodulin.com or call Customer Service at 1-844-UNITHER (1-844-864-8437).
REMISIconFEB2025
PAH=pulmonary arterial hypertension; WHO=World Health Organization.

